Measles
Edmonston strain:
Enders’ attenuated Edmonston strain. Edmonston wild-type (wt) measles virus (Parks et al., 2001). In 1954, the measles virus was isolated from an 11-year old boy from the US, David Edmonston, and adapted and propagated on chick embryo tissue culture (CE). The CE adapted strain, known as Edmonston A, was too virulent for vaccine purposes. The strain was attenuated by means of further passages on CE fibroblasts, resulting in a 2nd generation attenuated virus designated as Edmonston B. Again, the strain was too virulent to be applied on a large scale. Laboratories continued to pass Edmonston B on CE until a 3rd generation of more attenuated strains was developed. These strains, which are known by different names and differ from each other in the number of times the parent strain was passed on CE, provide the seeds for the vaccines now commercially available. The measles vaccines supplied through the World Health Organization’s programme on Immunization in the Americas are prepared from seeds derived from Edmonston B (EPI Newsl., 1980). GenBank Taxonomy No.: 11235
For more information on the various Edmonston strains
Schwarz strain:
Further passages of Edmonston A and B on chicken embryo fibroblasts (CEF) produced the more attenuated Schwarz and Moraten viruses, whose sequences have recently been shown to be identical (Combredet et al., 2003). The Moraten and Schwarz strains are highly genetically related, reflecting their common ancestry and similar passage history, and they are safe and effective for most children. Their use has dramatically reduced the incidence of measles, from over 100 million cases in the prevaccine era to approximately 31 million cases in 1997. However, fatal infections have been documented in immunodeficient children vaccinated with these strains (Valsamakis et al., 1999). GenBank Taxonomy No.: 132487
For more information on the various Schwarz strains
The Measles virus is monotypic, or in other words, there is only one serotype. The genetic differences among the various strains can cause variations in the clinical manifestations of infection. Since the Measles virus is monotypic, immunity against one strain can provide immunity against all strains. Even with various genotypes, the viral genomes have not deviated to produce multiple serotypes.
The Edmonston strain of measles virus isolated in cell culture in 1954 (7) became the progenitor for many live attenuated measles vaccines such as Moraten, Schwarz, Zagreb and Edmonston B (8). Several measles strains were also used for the development of measles vaccines in other countries such as Russia, China and Japan. All these attenuated strains are used around the world and despite the differences in methods employed for attenuation of the original viruses, which involve various cell culture systems, incubation temperatures, and number of passages, remarkable nucleotide sequence similarity has been found among the strains (9).
A live attenuated vaccine (CAM-70) derived from a Japanese isolate has been used in Brazil since 1982. National vaccination campaigns achieved more than 95% coverage after the Brazilian Measles Eradication Program was established in 1992, although some epidemic outbreaks have occurred after the introduction of the program, mainly in 1997 (2,10)…
CAM-70 vaccine was developed in 1970 from the Tanabe strain in Japan by adaptation to the chorioallantoic membrane (CAM) of chick embryos (12-15). This vaccine has been produced at Bio-Manguinhos/FIOCRUZ since the early eighties by a technology transfer from the Biken Institute, Japan.
Since 1963, when both an inactivated and a live attenuated vaccine (Edmonston B strain) were licensed for use in the United States, both the type of measles vaccine and the recommended age for measles vaccination have changed several times. After 1967 and 1975, the inactivated and the Edmonston B vaccine, respectively, were no longer distributed. A live, further attenuated vaccine (Schwarz strain) was first introduced in 1965, and a similar vaccine (Moraten strain) was licensed in 1968. These further attenuated vaccines cause fewer reactions than the Edmonston B vaccine, yet are equally effective. The Moraten vaccine is the vaccine used currently in the United States.
ROUVAX-Live attenuated vaccine against measles (Schwartz strain)
Each vaccine dose contains:
Lyophilizate:
– Hyperattenuated live measles virus
SCHWARZ strain . . . . . . . . . . . . . . . . . ….. . at least 1000 CCID50*
– Human albumin . . . . . . . . . . . . . . . . . . . . . . . . … q.s. for lyophilization
Solvent:
– Water for injections . . . . . . . . . . . . . . . . . . . . . . . . . . . . . q.s. 0.5 ml
*CCID50 = Cell culture infectious dose 50 percent.
This vaccine contains traces of neomycin.
This vaccine also contains lactose.
This vaccine is in conformity with WHO specifications.
M-M-R® II (MEASLES, MUMPS, and RUBELLA VIRUS VACCINE LIVE)
M-M-R* II (Measles, Mumps, and Rubella Virus Vaccine Live) is a live virus vaccine for vaccination against measles (rubeola), mumps, and rubella (German measles).
M-M-R II is a sterile lyophilized preparation of (1) ATTENUVAX* (Measles Virus Vaccine Live), a more attenuated line of measles virus, derived from Enders’ attenuated Edmonston strain and propagated in chick embryo cell culture; (2) MUMPSVAX* (Mumps Virus Vaccine Live), the Jeryl Lynn** (B level) strain of mumps virus propagated in chick embryo cell culture; and (3) MERUVAX* II (Rubella Virus Vaccine Live), the Wistar RA 27/3 strain of live attenuated rubella virus propagated in WI-38 human diploid lung fibroblasts.
The growth medium for measles and mumps is Medium 199 (a buffered salt solution containing vitamins and amino acids and supplemented with fetal bovine serum) containing SPGA (sucrose, phosphate, glutamate, and recombinant human albumin) as stabilizer and neomycin.
The reconstituted vaccine is for subcutaneous administration. Each 0.5 mL dose contains not less than 1,000 TCID50 (tissue culture infectious doses) of measles virus; 12,500 TCID50 of mumps virus; and 1,000 TCID50 of rubella virus. Each dose of the vaccine is calculated to contain sorbitol (14.5 mg), sodium phosphate, sucrose (1.9 mg), sodium chloride, hydrolyzed gelatin (14.5 mg), recombinant human albumin (≤0.3 mg), fetal bovine serum (<1 ppm), other buffer and media ingredients and approximately 25 mcg of neomycin. The product contains no preservative.
ATTENUVAX® (MEASLES VIRUS VACCINE LIVE)
ATTENUVAX* (Measles Virus Vaccine Live) is a live virus vaccine for vaccination against measles (rubeola).
ATTENUVAX is a sterile lyophilized preparation of a more attenuated line of measles virus derived from Enders’ attenuated Edmonston strain and propagated in chick embryo cell culture.
The growth medium for measles is Medium 199 (a buffered salt solution containing vitamins and amino acids and supplemented with fetal bovine serum) containing SPGA (sucrose, phosphate, glutamate, and human albumin) as stabilizer and neomycin. The cells, virus pools, fetal bovine serum, and human albumin are all screened for the absence of adventitious agents. Human albumin is processed using the Cohn cold ethanol fractionation procedure.
The reconstituted vaccine is for subcutaneous administration. Each 0.5 mL dose contains not less than 1,000 TCID50 (tissue culture infectious doses) of measles virus. Each dose of the vaccine is calculated to contain sorbitol (14.5 mg), sodium phosphate, sucrose (1.9 mg), sodium chloride, hydrolyzed gelatin (14.5 mg), human albumin (0.3 mg), fetal bovine serum (<1 ppm), other buffer and media ingredients and approximately 25 mcg of neomycin. The product contains no preservative.
ProQuad® Measles, Mumps, Rubella and Varicella Virus Vaccine Live
MMRV(Measles, Mumps, Rubella, Varicella)
Has been suspended.
ProQuad* is a combined attenuated live virus vaccine containing measles, mumps, rubella, and varicella viruses. ProQuad is a sterile lyophilized preparation of (1) the components of M-M-R*II (Measles, Mumps and Rubella Virus Vaccine Live): Measles Virus Vaccine Live, a more attenuated line of measles virus, derived from Enders’ attenuated Edmonston strain and propagated in chick embryo cell culture; Mumps Virus Vaccine Live, the Jeryl Lynn™ (B level) strain of mumps virus propagated in chick embryo
cell culture; Rubella Virus Vaccine Live, the Wistar RA 27/3 strain of live attenuated rubella virus propagated in WI-38 human diploid lung fibroblasts; and (2) Varicella Virus Vaccine Live (Oka/Merck), the Oka/Merck strain of varicella-zoster virus propagated in MRC-5 cells. The cells, virus pools, bovine serum, and human albumin used in manufacturing are all tested to provide assurance that the final product is free of potential adventitious agents.
ProQuad, when reconstituted as directed, is a sterile preparation for subcutaneous administration.
Each 0.5-mL dose contains not less than 3.00 log10 TCID50 (50% tissue culture infectious dose) of measles virus; 4.30 log10 TCID50 of mumps virus; 3.00 log10 TCID50 of rubella virus; and a minimum of 3.99 log10 PFU (plaque-forming units) of Oka/Merck varicella virus.
Killed Measles Vaccine (no longer used)
Severe local reactions to live measles virus vaccine following an immunization program.
Am J Public Health. 1983 August; 73(8): 899–900.

Edmonston-Zagreb (high-titer vaccine)
“In an experiment to find out of they could give high-potency Edmonston Zagreb (EZ) measles vaccine to babies as young as four months old [completing disregarding developmental neurology and lack of myelinization in the nervous system of babies] in order to overwhelm their natural maternal antibodies and replace them with vaccine-induced antibodies, medical “researchers” at the CDC and Johns Hopkins University injected thousands of babies in the Third World with the experimental vaccine that reportedly caused chronic immune suppression and the deaths of an unknown number of babies. Also, in the United States, with the help of Kaiser Permanente, more than 1500 six-month old black and Hispanic babies in inner city Los Angeles were “enrolled” in the experiment starting in June 1990. [ During the administration of president and ex-CIA director George Bush.] The study was halted in October 1991, after more than one year of genocidal activity, after repeated reports from vaccine trial sites in Africa that girl babies were dying in higher than expected numbers six months to three years after injection. [A less-than-admirable population control effort.] “–Leading Edge http://www.cco.net/~trufax/vaccine/0696.html Based on NVIC Vaccine Report 0696 Rec 9/3/96
Also see: MEASLES VACCINE EXPERIMENTS ON MINORITY CHILDREN TURN DEADLY
CDC Admits Informed Consent Violations – CDC director David Satcher admitted in a June 17 Los Angeles Times article that a National Institutes of Health (NIH) investigation of the 1990-91 Los Angeles study found that informed consent regulations had been violated because the parents were not told their babies would be injected with an experimental vaccine that had never been licensed by the FDA for use in America. Both Kaiser and the CDC have denied that any of the Los Angeles babies were harmed by the high potency EZ vaccine but did admit that one child, who received a standard potency EZ vaccine, died from a bacterial infection they maintain is unrelated to the vaccination.
The high potency EZ measles experiments began at four major sites in the mid-1980’s including Haiti, the Senegal, Guinea Bissau and Mexico. Other trials followed in Cameroon, Gambia, Bangladesh, Togo, Iran, New Guinea, Peru, Rwanda, Sudan, South Africa, Egypt, Philippines, Uzbekistan, Thailand, Zaire and Los Angeles. Primary funding came from the U.S. Agency for International Development (USAID) and the World Health Organization (WHO). In Haiti, infants were given the experimental vaccine at 10 to 500 times the usual dose levels. In a June 1996 article in Journal of Infectious Diseases, Johns Hopkins researchers report that infants with the highest antibody responses to high titer measles vaccine have the most profound immune suppression.
Warnings Ignored – The measles vaccine experiment was only stopped two years after the director of one of the African sites warned the WHO and CDC experiment leaders in April 1990 that African mortality data raised a red flag about the high titer EZ vaccine. His reports were first ignored and then discounted and he was replaced as a principal investigator. After his mortality data was dismissed as incorrect for more than a year, with support from colleagues at Harvard, he published the mortality data in The Lancet in October 1991. WHO then called for all the sites to submit mortality data for independent analysis. The CDC has stated that enrollment for the LA study was halted in October 1991.


EDMONSTON-ZAGREB MEASLES VACCINE PROJECT
Studies comparing Edmonston-Zagreb (EZ) measles vaccine with Schwartz measles vaccine in young infants (four to nine months of age) have been conducted in several developing countries, including Guinea-Bissau, Senegal, Haiti, Gambia, Togo, and the Philippines.
These studies revealed high seroconversion rates with EZ vaccine even in the presence of high maternal measles antibody. Several of these studies that used high-dose (4.84 In TCID50) EZ measles vaccine found increased mortality predominantly or exclusively among African female infants immunized with high-dose EZ measles vaccine. In these cases, death occurred approximately one year after vaccination. The causes of these deaths are common causes of infant and child mortality in Third World settings, e.g., respiratory infections, diarrhea and dehydration. Data from these, as well as other studies which did not find higher mortality post-vaccination, were reviewed during a two-day meeting organized by the Expanded Programme on Immunization (EPI), World Health Organization (WHO), in February 1991. Principal investigators of the major clinical trials of EZ vaccine and an international panel of experts attended the meeting and reviewed the data. The panel felt that the probability that the higher mortality was due to the vaccine was very low. Among the reasons cited was the lack of biologic plausibility, particularly in reference to the sex-specific findings.
DIPLOVAX HDC 4.0 –Freeze-dried measles vaccine produced from the Edmonston-Zagreb strain which has been further attenuated in human diploid cells.
The vaccine contains live, attentuated measles virus of the Edmonston-Zagreb strain, propagated in human diploid cells. Each dose of 0.5 mL contains at least 104 CCID50 (cell culture infective doses) of live measles virus. The vaccine is stabilized with a 10% gelatin-sorbitol stabiliser. The freeze-dried vaccine is reconstituted with 0,5 mL Sterile Water for Injections per dose.
…is prepared from live attenuated strains of Edmonston-Zagreb Measles virus propagated on human diploid cell culture, L-Zagreb Mumps virus propagated on chick embryo fibroblast cells and Wistar RA 27/3 Rubella virus propagated on human diploid cell culture.
The reconstituted vaccine contains, in single dose of 0.5 ml. not less than
1000 CCID50 of Measles virus
5000 CCID50 of Mumps virus
1000 CCID50 of Rubella virus.
Diluent : Sterile water for injection.
The vaccine meets the requirements of USP and WHO when tested by the methods outlined in USP and WHO, TRS 840 (1994).
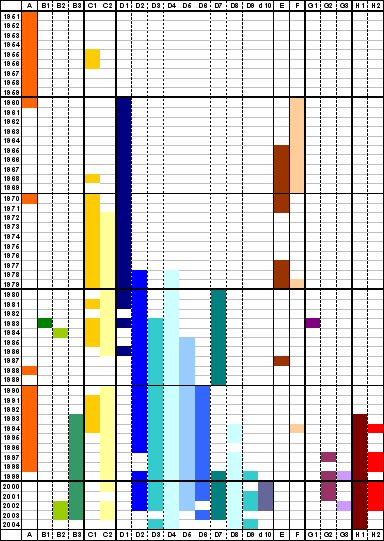
Measles virus are divided into 21 genotypes
Review of the temporal and geographical distribution of measles virus genotypes in the prevaccine and postvaccine eras (Virology Journal 2005, 2:87)
Although measles virus (MV) is serologically monotypic, the genetic characterization of wild-type viruses has identified eight clades (A – H), which have been divided into 22 genotypes and one proposed genotype. Clades B, C, D, G and H each contain multiple genotypes (B1 – 3, C1 – 2, D1 – 10, G1 – 3, H1 – 2) while clades A, E and F each contain a single genotype (A, E, F) [1,2]. The sequences of the vaccine strains indicate that the wild type viruses from which they were derived were all members of genotype A. All measles genotypes can be neutralized by serum from vaccinated persons in vitro, although with varying efficiency [3,4]. There are no known biological differences between viruses of different genotypes. Specific measles genotypes are not associated with differences in severity of disease, likelihood of developing severe sequela such as subacute sclerosing panencephalitis or inclusion body encephalitis, or variability in sensitivity of laboratory diagnosis.
Review of the temporal and geographical distribution of measles virus genotypes 1951 – 2004
Although measles virus (MV) is serologically monotypic, the genetic characterization of wild-type viruses has identified eight clades (A – H), which have been divided into 22 genotypes and one proposed genotype. Clades B, C, D, G and H each contain multiple genotypes (B1 – 3, C1 – 2, D1 – 10, G1 – 3, H1 – 2) while clades A, E and F each contain a single genotype (A, E, F). The sequences of the vaccine strains indicate that the wild type viruses from which they were derived were all members of genotype A.
There are no known biological differences between viruses of different genotypes. Specific measles genotypes are not associated with differences in severity of disease, likelihood of developing severe sequela such as subacute sclerosing panencephalitis or inclusion body encephalitis, or variability in sensitivity of laboratory diagnosis.
Analysis of the variability in the nucleotide sequences of wild-type MVs has enabled the use of molecular epidemiologic techniques for measles surveillance. Genetic characterization of viral isolates or RT-PCR products is the only laboratory test that can differentiate between vaccine-associated cases and wild-type infection.
In 1998, the World Health Organization (WHO) recommended a standard protocol for the designation of measles genotypes. The minimum amount of sequence data required to assign a virus to a genotype are the 450 nucleotides encoding the carboxy terminus of the N protein. The entire sequence of the coding region of the H gene should be obtained from representative isolates.
The purpose of this summary is to collate all available reports of MV genotypes and to standardize the published genotype nomenclature, according to the current WHO criteria, with the aim of giving a comprehensive overview of the distribution of MV genotypes in the prevaccine and postvaccine eras.
Summary of distribution of MV genotypes from the prevaccine era to 2004
Genetic Diversity of Wild-Type Measles Viruses: Implications for Global Measles Elimination Programs
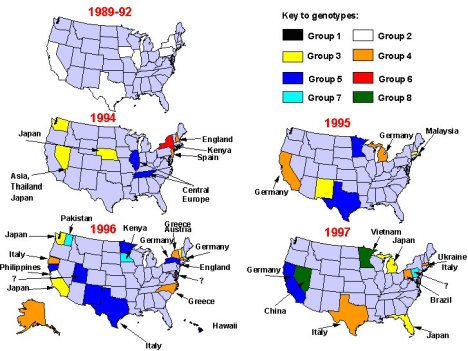

Figure 4. Change in genetic groups of measles viruses associated with U.S. cases and outbreaks between 1988 and September 1997. Arrows indicate sources of virus, if known.
Filed under: Measles | Tagged: strains | Comments Off on Name That Strain