Rubella
Global Measles and Rubella Laboratory Network, January 2004-June 2005
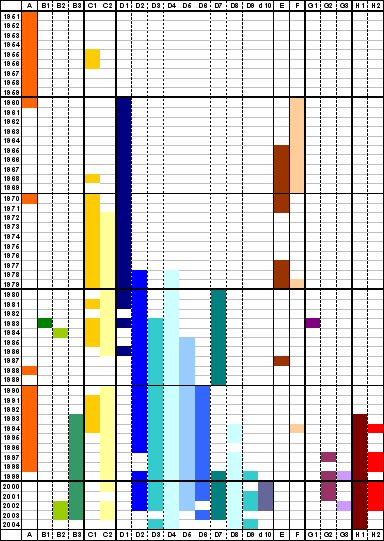
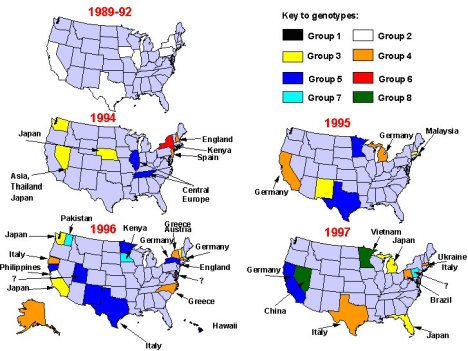
Seven genotypes and three additional provisional genotypes of rubella virus are recognized by WHO (Figure 2). These genotypes are classified into two clades (i.e., groups of similar genotypes), designated 1 and 2; clade 2 viruses have not been found circulating in the western hemisphere. Although knowledge concerning the geographic distribution of rubella genotypes has progressed substantially since 2003, the genotypes of rubella viruses present in many countries and regions remain unknown.

Global strains of rubella virus
Based on analysis of the viral E1 gene, there are two genotypes of rubella virus: Genotype I is present in Europe, North America, and Asia; Genotype II is present in China, Israel and Korea and appears to co-exist with Genotype I viruses in these countries 14. While these genotypes differ by 7-11% at the nucleotide level, they differ by less than 3% at the amino acid level and are of the same serotype. Therefore, the biological significance of the evolution and maintenance of two genotypes is unclear. Following the widespread use of rubella vaccine in developed countries from the 1970s onward, a change in rubella strains occurred in which an
intercontinental genotype clade of Genotype I present in Europe, North America, and Japan replaced the geographic clades from each continent. There are still large areas of the world from which no rubella virus isolates have been analyzed (Africa, Australia, and most of mainland Asia) and thus there could be additional genotypes as well as unrecognized geographic clades of Genotypes I and II. It will be important to obtain a selection of rubella virus isolates from these regions before or concurrent with widespread introduction of rubella vaccination so that the pre-existing endemic virus genotypes can be identified…(Frey TK, Abernathy ES, Bosma TJ et al. Molecular analysis of rubella virus epidemiology across three continents, North America, Europe, and Asia, 1961-1997. Journal of Infectious Disease 1998;178:642-650).
EVOLUTION AND DISTRIBUTION OF RUBELLA VIRUS GENOTYPES
Rubella virus is a sole member of Rubivirus group of Togaviridae and its genotypes has been classified in 2004 at WHO meeting. They are genotype 1B, 1C, 1D, 1E, 1F , 2A and 2B as confirmed and 1a, 1g and 2c as provisional. They have unique chronological and geographical characteristics. Genotype 1a worldwide distributed in 1960s and 70s, however almost disappeared since 1980. Genotype 1B mainly has distributed in Europe and genotype 1C in North and South American continents. Genotype 1D has distributed mainly in Asia and genotype 1F is restricted in China. Genotype 1E looks to be derived from genotype 1D and recently becomes to be predominant circulating one worldwide since 1997. Genotype 1g looks to be derived from genotype 1B and distributes in Europe and Americas. Genotype 2A was restricted in China and 2B in Eurasia and Africa. Genotype 2c was found in Russia. Molecular epidemiological study of rubella virus genotype could reveal the transportation of rubella virus from a country to another country. It will greatly help to make a effective plan of rubella immunization program to eliminate and eradicate for a certain country.
Ancestor dating analysis resulted in 1942-46 for virus strains in genotype 1 and 1840 for those in genotype 2.
By these analysis shift of major prevailing genotype of rubella virus may happened in the history of this diseases, at least from genotype 2 to genotype 1 and genotype 1a to genotype 1E.
As rubella has no relating animal viruses as far as studied, this evolution and emergence is very curious to be known in the future.
Mapping of Genetic Determinants of Rubella Virus Associated with Growth in Joint Tissue
J Virol. 2000 January; 74(2): 796–804. PMCID: PMC111599
Rubella virus (RV), the etiologic agent of German measles, belongs to the family Togaviridae and is the only member of the genus Rubivirus. Natural infection in childhood causes a systemic illness characterized by a short-lived maculopapular rash and mild fever (50). The disease is generally benign, and infection is often asymptomatic. It is the teratogenic potential of rubella that brought the virus to the forefront of public health interests and provided the impetus for isolation of the virus and subsequent vaccine development (35, 49). The current vaccine strain RA27/3 has been very effective in reducing the incidence of congenital rubella syndrome in North America, where it is given to all children between 12 and 18 months of age. However like the wild-type strains and the earlier vaccine strain, HPV77/DE5, it is reported to be associated with acute and late-onset joint and neurological symptoms (20, 46, 47, 50).
The association of RV with acute, transient joint manifestations, after both natural infection and vaccination, has been recognized for many years (14, 23, 34, 46, 47). Rubella-associated arthritis (RAA) is usually short-lived, although a number of patients go on to develop chronic or recurrent pauci- or polyarticular symptoms which can persist for some time (7, 8, 22, 43, 45, 47). Studies to define the mechanism of pathogenesis of RAA have been limited by the fact that humans are the only natural host for RV and there is presently no animal model of infection. However, the frequency and intensity of clinical symptoms reported for wild-type (wt) and vaccine strains correlate directly with the ability of the infecting strain to propagate in organ cultures of human synovial tissue, suggesting that tropism for joint tissue is a measure of viral arthritogenicity (31). Although RV strains are genetically around 98% homologous, they display striking phenotypic variation in growth characteristics and plaque morphology as well as tropism for joint tissue (9, 31). The wt strains, such as Therien, which have the highest association with persistent joint symptoms (30%) (47), commonly replicate to titers of 106 to 107 PFU/ml in organ cultures of human joint tissue, comparable to the yields from the most permissive cell lines for RV. The vaccine strain RA27/3, which is associated with much lower levels of recurrent arthritis (4%) (47), is severely restricted in these cultures and does not attain titers greater than 103 PFU/ml. However RA27/3, like the wt Therien strain, was found to persist in joint culture for over 3 months (31). In contrast, no replication of the European vaccine strain, Cendehill, was detected in human joint tissue in this study. Cendehill strain is reported to have a very low association with acute arthritis and none with chronic joint manifestations (4). These results indicate a correlation between the arthrotropism of a specific RV strain and its ability to induce joint symptoms and lends support to the hypothesis that recurrent RAA is triggered by reactivation of virus which has established a persistent infection in the joint.
The Vaccines:
Soon after the rubella virus was first isolated in tissue culture in 1962, several live-attenuated vaccine strains were developed. HPV-77 (duck embryo), HPV-77 (dog kidney) and Cendehill (rabbit kidney) strains were originally licensed in the USA between 1969 and 1970. These vaccines were replaced in 1979 by RA 27/3 (human diploid fibroblast), which produces a strong immune response (similar to natural infection) of 95% or more. While rubella immunity induced by vaccination has been reported to persist for at least 16 years and probably to be lifelong, other recent data indicate that this immunity may wane after 8 years of age. [3] Rubella vaccine is usually offered in combination with measles (MR) or measles and mumps (MMR) vaccines. [1,5] This combination offers the same high levels of immunogenicity and safety as does its individual components. Most of the currently licensed vaccines are based on a live, attenuated strain of rubella virus known as RA 27/3. The vaccines are administered subcutaneously. To avoid interference with possible remaining maternal antibodies the vaccine is usually given at the age of 12-15 months. Attempts to develop killed virus vaccines or sub-component vaccines against rubella have not been successful…
MERUVAX* II
Wistar RA 27/3 strain of live attenuated rubella virus propagated in WI-38 human diploid lung fibroblasts.
BIAVAX® II (Rubella and Mumps Virus Vaccine Live) is a live virus vaccine for immunization against rubella (German measles) and mumps. BIAVAX II is a sterile lyophilized preparation of the Wistar RA 27/3 strain of live attenuated rubella virus grown in human diploid cell (WI-38) culture
PROQUAD -MMRV(Measles, Mumps, Rubella, Varicella)
Has been suspended.
ProQuad* is a combined attenuated live virus vaccine containing measles, mumps, rubella, and varicella viruses. ProQuad is a sterile lyophilized preparation of (1) the components of M-M-R*II (Measles, Mumps and Rubella Virus Vaccine Live): Measles Virus Vaccine Live, a more attenuated line of measles virus, derived from Enders’ attenuated Edmonston strain and propagated in chick embryo cell culture; Mumps Virus Vaccine Live, the Jeryl Lynn™ (B level) strain of mumps virus propagated in chick embryo
cell culture; Rubella Virus Vaccine Live, the Wistar RA 27/3 strain of live attenuated rubella virus propagated in WI-38 human diploid lung fibroblasts; and (2) Varicella Virus Vaccine Live (Oka/Merck), the Oka/Merck strain of varicella-zoster virus propagated in MRC-5 cells. The cells, virus pools, bovine serum, and human albumin used in manufacturing are all tested to provide assurance that the final product is free of potential adventitious agents.
ProQuad, when reconstituted as directed, is a sterile preparation for subcutaneous administration.
Each 0.5-mL dose contains not less than 3.00 log10 TCID50 (50% tissue culture infectious dose) of measles virus; 4.30 log10 TCID50 of mumps virus; 3.00 log10 TCID50 of rubella virus; and a minimum of 3.99 log10 PFU (plaque-forming units) of Oka/Merck varicella virus.
M-M-R® II (MEASLES, MUMPS, and RUBELLA VIRUS VACCINE LIVE)
Note: “….contains attenuated live measles and mumps viruses propagated in chick embryo cell culture, plus “the Wistar RA 27/3 strain of live attenuated rubella virus propagated in WI-38 human diploid lung fibroblasts.”(1) Principal studies published in the American Journal of Diseases of Children and the American Journal of Epidemiology, reveal that the rubella strain was cultured from an aborted human fetus.(2,3) In addition, the growth medium for the three live viruses that are needed to produce the MMR vaccine is a buffered salt solution “supplemented with fetal bovine serum.”(4) Other ingredients include sucrose, phosphate, glutamate, recombinant human albumin, sorbitol, hydrolyzed gelatin stabilizer, and approximately 25 mcg of neomycin (an antibiotic).(5) The MMR vaccine does not contain a preservative. In fact, according to the FDA, MMR-II never contained thimerosal, a potentially dangerous chemical used in some vaccines.(6) However, trace amounts of mercury were detected in an earlier MMR formulation.”
Genomic sequence of the RA27/3 vaccine strain of rubella virus.
Arch Virol. 1997; 142(6):1165-80. PMID: 9229006 [PubMed – indexed for MEDLINE]
The sequence of the genome of the RA27/3 vaccine strain of rubella virus (RUB) was determined. In the process, several discrepancies between the previously reported genomic sequences of two wild RUB strains (Therien and M33) were resolved. The genomes of all three strains contain 9762 nucleotides (nts), exclusive of the 3′ poly A tract. In all three strains, the genome contains (5′ to 3′), a 40 nt 5′ untranslated region (UTR), an open reading frame (ORF) of 6348 nts that encodes nonstructural proteins, a 123 nt UTR between the two genomic ORFs, a 3189 nt ORF that encodes the structural proteins, and a 62 nt 3′ UTR. The 5′ end of the subgenomic RNA was found to correspond to a uridine residue at nt 6436 of the genomic RNA. At the nucleotide level, the sequence of the three strains varied by 1.0 to 2.8%, while at the amino acid level, the sequence varied by 1.1 to 2.4% over both ORFs. The RA27/3 sequence will be of use in identification of the determinants of its attenuation, in vaccine production control and in development of second generation RUB vaccines based on recombinant DNA technology.
A comparative field evaluation of three live, attenuated rubella virus vaccines.
Am J Public Health. 1971 January; 61(1): 152–156.
*Before aborted fetal cell lines were used

Countries using rubella vaccine, by WHO region, as of December 1999* (pg 69)
Rubella and congenital rubella syndrome: global update

Filed under: Measles | Tagged: Rubella | Comments Off on Rubella Strains